From January 1st 2016 to September 30th 2019, MultiMed Engineers participated in the BD2Decide Project — Big Data and models for personalized Head and Neck Cancer Decision support.
Cancers of the Head and Neck Region (HNC) are the 6th more deadly cancers worldwide: in Europe ~150.000 new cases are detected and ~70.000 patients die every year. The main reasons for high mortality are the fact that the majority of cases are diagnosed in advanced Stage and the intrinsic heterogeneity of such tumors.
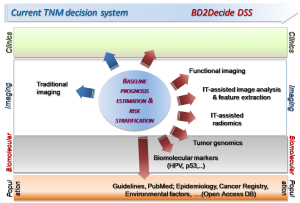
At present the only adopted treatment decision method is based on TNM (Tumor-lymph-Nodes-Metastasis) prognostic system, that considers only a few risk factors such as smoking, alcohol abuse and more recently HPV. The TNM system is therefore inadequate to capture the patient-specific biomolecular characteristics of the tumor.
HNC treatments can have hard impact on patient’s aesthetics and functionalities and, due to their toxicity, can cause severe morbidity and greatly deteriorate patient’s quality of life. A more precise prognostic prediction than the current TNM system is needed that allows implementing the first-line treatment that maximizes the therapeutic result and minimizes the impacts of therapy.
BD2Decide DSS is intended to provide clinicians with the “means” and all the necessary information to tailor treatment and care delivery pathway to each and any HNC patient during their usual practice, in contrast to current “one-size-fits-all approach”. BD2Decide realized and validated an Integrated Decision Support System that links population-specific epidemiology and behavioral data, patient-specific genomic, pathology, clinical and imaging data with big data techniques, multiscale prognostic models. Advanced graphical visualization tools have been developed for prognostic data disclosure and patient co-participation to the selected treatment. BD2Decide intent is to improve the clinical decision process, uncover new patient-specific patterns that can improve care, and create a virtuous circle of learning.
A multicentric clinical study with more than 1.000 patients has been used to validate the system.
Besides MultiMed Engineers, the Project Consortium included Azienda Ospedaliero-Universitaria di Parma (IT, coordinator), Stichting VU/VUMC (NL), Universität Düsseldorf (DE), Fondazione IRCCS Istituto dei Tumori Milano (IT), Universidad Politecnica de Madrid (ES), Fraunhofer Gesellschaft (DE), All-in-Image Ltd. (IL), Politecnico di Milano (IT), Athens Technological Centre S.A. (EL), Maastro Clinic (NL), Università di Parma (IT).
MultiMed Engineers has been mainly involved in Task 8.3 — Health technology and assessment of the usability of the system and in Workpackage 9 — Exploitation strategy and actions for maximizing project impact.
Project web site: www.bd2decide.eu

This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 689715
 MultiMed Engineers
MultiMed Engineers