Since July 1st 2024, MultiMed Engineers participates in the PROTECT-CHILD Project — A PRivacy-prOTecting Environment for Child Transplants health-related and genomic data integration in the European Reference Network.
Paediatric transplantation (PT) is the only curative therapeutic procedure for most end-stage diseases affecting different organs and body systems, producing a transformative impact on the health and quality of life (QoL) of children. Every year more than 5000 children receive either a solid organ transplant (SOT) or a haematopoietic stem cell transplant (HSCT) in Europe. Both SOT and HSCT offer the chance of a cure, but at the same time raise the risk of treatment-related mortality and long-term morbidities. Long-term survival and optimum quality of life are fundamental requirements after child transplant due to their longer and more active life expectancy and associated requirements as compared to adults.
The integration of all kinds of available data, including unstructured real-world data and genomic data, is crucial to foster optimal outcomes for transplants in children, especially for the most complex and rare, for which the clinical evidence is limited.
The objective of PROTECT-CHILD is to improve the treatment and follow-up of transplanted children by exploiting ERN TransplantChild’s wealth of data, integrating it with existing and newly generated genome and methylome study data from a prospective cohort of transplanted children that will inform on pharmacogenetics, immune response to IS drugs and to infections, and provide specific epigenetic signatures for liver and kidney child transplants. This would lead to better targeted therapies and improved follow-up, to prevent and avoid treatment toxicities and side effects, new diseases and finally ensure a better quality of life. PROTECT-CHILD will be experienced on two transplant cases: liver and kidney, involving 200 patients in 4 European hospitals.
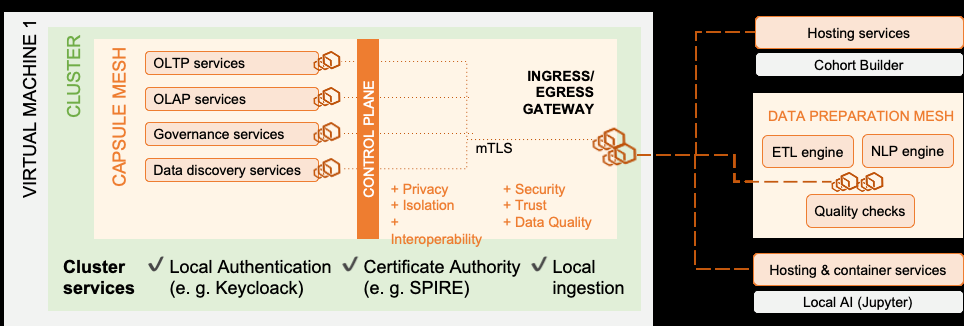
The project will tackle the challenge of conceiving, developing, and validating a data space for the TransplantChild ERN, aimed at supporting the Network’s needs for secondary use of health data, across its participating Centres of Excellence, located in different European Countries.
In general terms, the project will develop a multi-source, multi-standard, data sharing infrastructure and governance approach, which will be (i) fully aligned with the principles envisioned in the EHDS regulation proposed by the Directorate-General for Health and Food Safety of the European Commission; (ii) synergistic with the Genomic Data Infrastructure (GDI) of the 1+Million Genomes (1+MG) initiative; and (iii) coordinated with the ELIXIR infrastructure for discovering, sharing, and analysing large datasets in the life science domain, in line with the European Open Science Cloud environment.
The Project Consortium is coordinated by UNIVERSIDAD POLITECNICA DE MADRID (ES) and includes — in addition to MultiMed Engineers — the following Partners: PREDICTBY RESEARCH AND CONSULTING S.L. (ES), LAEGEMIDDELSTYRELSEN (DK), F. HOFFMANN-LA ROCHE AG (CH), INSTITUT ZA EKONOMSKA RAZISKOVANJA (SI), INSTITUTET FOR FREMTIDSFORSKNING
FORENING (DK), FUNDACIO INSTITUT D’INVESTIGACIO BIOMEDICA DE BELLVITGE (ES), PHILIPS MEDICAL SYSTEMS NEDERLAND BV (NL), HEINRICH-HEINE-UNIVERSITAET DUESSELDORF (DE), TILBURG UNIVERSITY- UNIVERSITEIT VAN TILBURG (NL), DEDALUS ITALIA S.P.A. (IT), FONDAZIONE ITALIANA SCLEROSI MULTIPLA – FISM ETS (IT), AGENZIA REGIONALE PER LA SALUTE ED IL SOCIALE (IT), ISERUNDSCHMIDT GMBH (DE), BETTER PROGRAMSKA OPREMA DOO (SI), STICHTING HET NEDERLANDS KANKER INSTITUUT-ANTONI VAN LEEUWENHOEK ZIEKENHUIS (NL), FACHHOCHSCHULE ST. POLTEN GMBH (DE), UNIVERZITETNI KLINICNI CENTER LJUBLJANA (SI), UNIVERSITEIT UTRECHT (NL), MEDTRONIC IBERICA SA (ES), FUNDACIO HOSPITAL UNIVERSITARI VALL D’HEBRON – INSTITUT DE RECERCA (ES), SPLOSNA BOLNISNICA CELJE (SI), ORTOPEDSKA BOLNISNICA VALDOLTRA (SI), ETHNIKO KENTRO EREVNAS KAI TECHNOLOGIKIS ANAPTYXIS (GR), PHILIPS GMBH (DE), UDG ALLIANCE (CH).
MultiMed Engineers, is involved in the following project Work-packages:
- WP1: Coordination
- WP2 Codesign and multi-stakeholders’ requirements
- WP3 Big Data Infrastructure for massive data management
- WP8 Pilot studies preparation and set-up
- WP9 Genome sequencing and analysis of PROTECT-CHILD pilot use cases
- WP10 Communication, dissemination and exploitation of results
- WP11 Ethical, Legal and Social Issues Assessment
Project Website: https://protect-child.eu/

This project has received funding from the European Union’s Horizon Europe research and innovation programme under grant agreement No 101137423
 MultiMed Engineers
MultiMed Engineers